| Back |
|---|
When mains power is not available or not allowed such as on Field Days, or when “back packing” with portable equipment, then some form of battery storage of electric power, (often topped up from a petrol or diesel generator), is often used. For cheapness and availability, though not necessarily for lightness, the 12 Volt car battery or accumulator, sometimes with a “gel” electrolyte, is often used. This article attempts to explain how the chemistry of the re-chargeable Lead-Acid cell produces copious quantities of current at a nearly constant voltage.
Chemistry Lesson
Strictly speaking a battery is a collection of cells, in this case connected in series. I.e. Six 2 Volt cells making a 12V battery. It is called a Lead-Acid battery because its main constituents are Lead, (Chemical symbol Pb), and Sulphuric Acid, (chemical formula H2SO4), diluted with water, (formula H2O). (Water is called the solvent and what is dissolved in it, (sulphuric acid), is called the solute). A small proportion of both these last ingredients self-ionise into separate and oppositely charged ions. Water self ionises into two Hydrogen ions, (H+) and an Oxygen ion, (O- -). (The oxygen ion has two negative charges to balance the two singly charged hydrogen ions). The high dielectric constant of water, (about 80 at DC), enables its molecules to “slip between” the components of other, (solute), molecules scarcely noticed, enabling them to also partially separate and ionise This can be envisaged as; “any electric field within the solute molecule is hardly affected by the intervening water molecule”. The high dielectric constant of water and its ability to ionise and re-form accounts for why it is such a good solvent for so many materials. The sulphuric acid molecule in solution ionises into two H+ ions and an SO4- – ion, thus keeping the overall molecule electrically neutral.
Battery construction
Each cell in its fully charged state consists basically of a plate of fairly pure Lead, (chemical symbol Pb), and a plate of compressed Lead peroxide, (PbO2), immersed in dilute, (though fairly strong), sulphuric acid, (H2SO4). In order to decrease the internal resistance of each cell so that a large current may be drawn, a number of plates of each type are connected in parallel separated by insulating plastic separators to prevent the Lead and Lead peroxide plates coming into contact.
Electro-Chemical action
A fully charged Lead-Acid cell is shown symbolically in figure 1.
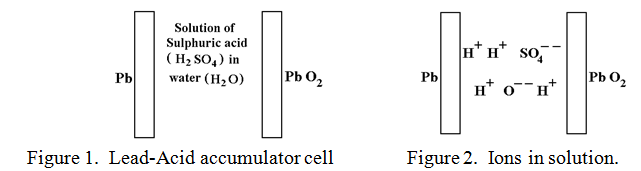
Pure Lead and Lead peroxide are not normally attacked by sulphuric acid, although ordinary Lead oxide, (PbO) is. The ionised sulphuric acid and water molecules are depicted in figure 2.
The powerful and corrosive SO4- – ions are itching to attack the pure Lead plate and convert it into Lead sulphate, (PbSO4), but as soon as the SO4- – ions have attacked a few Lead atoms the negative charge that the ions are carrying are transferred to the Lead plate which becomes negatively charged and this repels any further approach by SO4- – ions. It would be so much easier for this attack to succeed if the negative charges, (electrons), could be removed from the Lead plate as soon as they arrived. Then the lead plate would be converted to Lead sulphate quite easily.
In a similar manner, the solution of Hydrogen ions, (H+) would love to remove an Oxygen atom from the Lead peroxide to form the very stable compound water, (H2O), but their positive charge, being transferred in like manner to the Lead peroxide plate deters any further approach by positive H+ ions. If only there were a way of feeding electrons with their negative charge onto the Lead peroxide plate to neutralise this positive charge then the “water forming” reaction could go ahead. There is! This is exactly what happens when a wire is connected between the Lead plate and the Lead peroxide plate as indicated in figure 3.
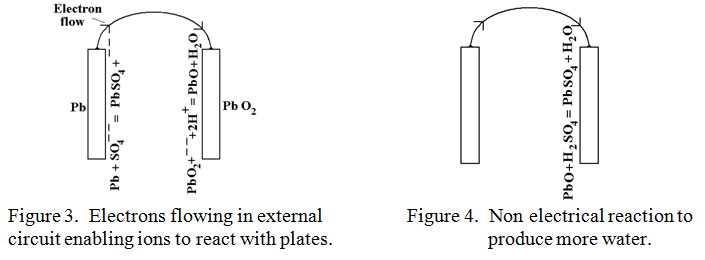
Electrons flow out of the Lead plate, (which is now uninhibited from accepting more sulphate ions), and into the Lead peroxide plate where they neutralise the positive charge. The Lead peroxide plate is now uninhibited from accepting more H+ ions and releasing one of its oxygen atoms to make water, (H2O), leaving behind ordinary Lead Oxide, PbO. This then succumbs to a sulphuric acid attack in a purely chemical, (i.e. non electrical) reaction to produce more water as follows:-
H2SO4 + PbO —-> PbSO4 + H2O This is shown diagrammatically in figure 4.
Complete discharge of the cell has occurred when both plates have been converted to Lead sulphate and the sulphuric acid has been further diluted by water in the above reaction.
Practical Considerations
I have described plates of pure Lead and Lead peroxide, in explaining the reactions. Actually, pure Lead is too soft to withstand a rough physical environment so it is alloyed with a small proportion of Antimony which is relatively inert and plays no part in the chemical process. The alloy is cast into a grid form and a “spongy Lead paste” is pressed onto the grid spaces. Similarly, the Lead peroxide plate is also made of a Lead Antimony alloy grid which is coated in an impervious layer of Lead peroxide before a spongy Lead-peroxide paste is pressed into its grid spaces.
The mechanism of converting chemical energy into electrical energy has been described, but the re-charging process, (converting electrical energy into chemical energy), is exactly the opposite, and hundreds of discharge-recharge cycles can be completed before a Lead Acid battery looses its capacity. This usually occurs because eventually the spongy Lead sulphate which fills the spaces in the plates when the cell is discharged gets replaced by a hard white form of lead sulphate which cannot easily be converted back to its original state. The cell should therefore never be left for any length of time in a discharged condition. When in good condition and fully charged, each cell has an emf of about 2.05 Volts, and the Specific Gravity of the acid is about 1.25 When almost completely discharged, and the emf has fallen to 1.8 Volts and the Specific Gravity has fallen to 1.1, it should then be recharged. At moderate charge rates, the water is not electrolysed into oxygen and hydrogen but is converted back into sulphuric acid and Lead peroxide. Thus there is virtually no “gassing” during recharging at low charge rates. The cell can therefore be sealed against spillage. A thixotropic agent is sometimes added to the acid as an aid in this respect giving rise to the so-called “Gel cell”. The Lead Acid cell is one of the most reliable and abuse tolerant of electrical storage cells.
| Back |
|---|
- PRAECEPTOR
