AB INITIO
| Back |
|---|
This month we take a slightly tongue in cheek look at the units which we all use in amateur radio such as Volts, Amps, Watts, metres, kilograms etc. Units have what are known as “dimensions” which, at their most fundamental, can usually be expressed in terms of length, mass and time, giving the various “systems of units” their names. The size of a “Unit” is chosen so that it can be easily compared and easily reproduced. Its value does not change with time. A modern unit is usually internationally accepted together with its abbreviation symbol. Nowadays, in science we mainly use “SI” units, (explained later), but it wasn’t always so.
In Isaac Newton’s day in Britain and much of Europe there were three main units for length, mass and time, and these were the foot, the pound and the second. With these he did all his excellent work on the laws of motion. These units remained good for 300 years, and we gave them to the world. They were of convenient size for every-day use, (like buying groceries), and also for scientific use. Their size was somewhat arbitrary but convenient. The foot, the length of a man’s foot, and its sub-division, the inch, (the length of a man’s thumb), the pound, a convenient weight for groceries, (and the Pound Sterling, the value of a pound weight of silver). The second was less arbitrary as it was related to the minute, the hour, and the mean length of a (solar) day. The units of the “foot-pound-second system” were formalised in the “Weights and Measures Act” of 1878 and eventually became known as the “Imperial System of Units”, though the Americans didn’t and still don’t like this term. Which set of units you use in no way affects the fundamental laws of physics. The principles remain the same. You can even invent your own system of units, say, a “Furlong Ton Fortnight” system, provided you use consistent numerical factors.
Then the French got involved and, with Napoleonic pride, felt that the size of the units should be less arbitrary. They felt that a length similar to a yard would be convenient, so they came up with a new unit of length, (of just over a yard), which they called the metre. This was originally defined as “one ten millionth part of the line of longitude passing through Paris and extending from the North Pole to the equator”. For convenience for comparing this length with various sub-standards, they made a platinum-iridium bar of this calculated length which they kept at a constant temperature in Paris. It looked remarkably similar to the “Standard Yard Bar” kept in London. However, they got their calculated distance from the North Pole to the equator wrong but still relied on their platinum bar. From the metre were derived the Tonne, (weight of a cubic metre of water), the Litre, one thousandth of a cubic metre, and the weight of one litre of water at 3.980C, (the temperature at which it is most dense), the kilogram. This led eventually to the older and now obsolete “centimetre gram second”, (CGS), system of scientific units but is still related to the modern SI system. These days the units are defined quite differently and with much greater precision., but are still of almost exactly the same size.
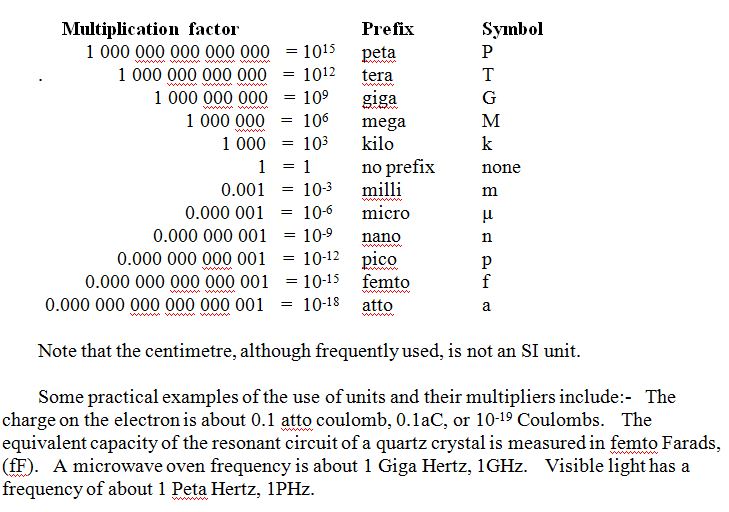
SI units are those of the “International System of Units” adopted in 1960 by the “Conférence Générale des Pois et Measures”. The primary units in this system are the metre, the kilogram and the second, (usually referred to as the mks system), and secondary units are: the Ampere, Kelvin, mole and candela. There is also a host of other “derived” units which include most of the electrical units. To all of these there are SI recognised multipliers. As stated earlier, units have “Dimensions”, and this fact can be useful when checking the voracity of scientific equations. Each side of an equation must be dimensionally equal in addition to any numerical correctness.

PRAECEPTOR
| Back |
|---|
